Selected papers: Ferris, S.H., et al., Positron emission tomography in the study of aging and senile dementia, Neurobiol. Aging, 1, 127-131: 1980; de Leon, M.J., et al., Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET), Proc. Natl. Acad. Sci., 98, 10966-10971: 2001. Mosconi, L., et al., FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease, Eur. J. Nucl. Med., 36(5), 811-822: 2008.
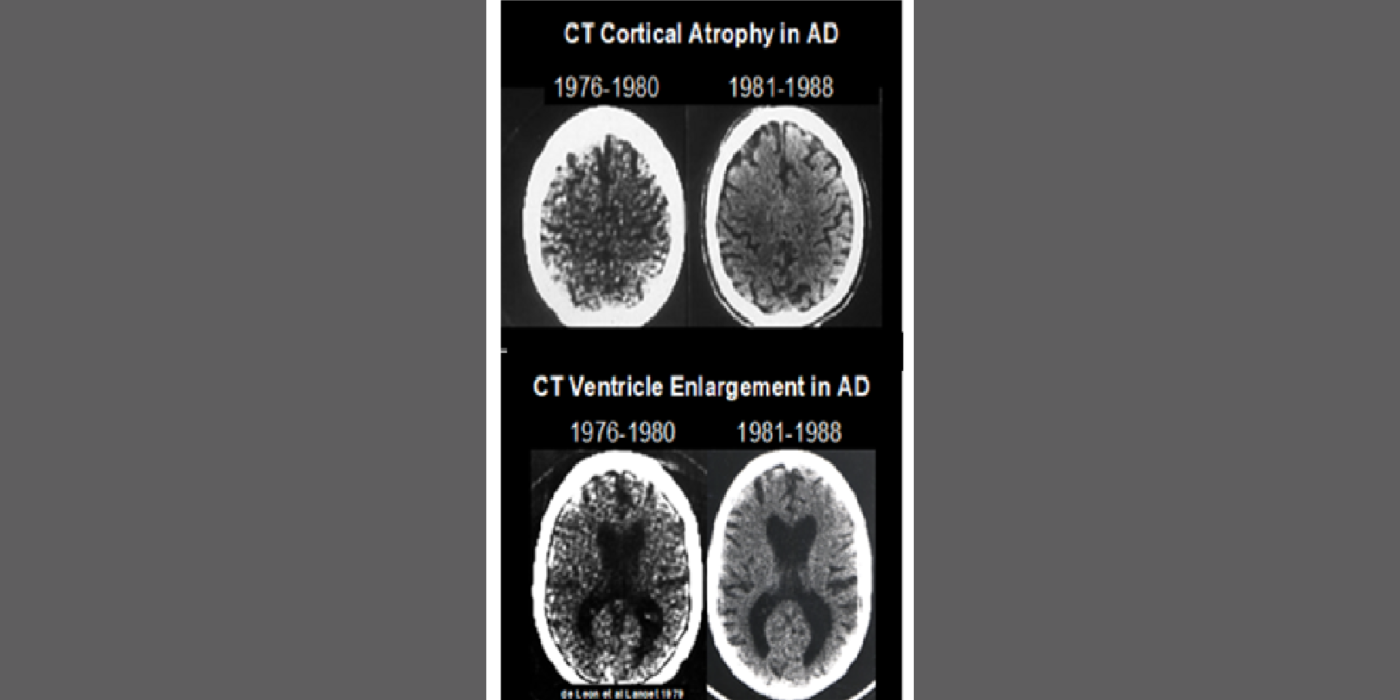
The team’s anatomical studies identified age-related periventricular white matter lesions on CT and (later) MRI scans. The team developed the first white-matter rating scale and showed these CT lesions were clinically associated with hypertension and, at pathology, with microvascular pathology. This work flourished in the MRI era.
Selected papers: George, A.E., et al., Leukoencephalopathy in normal and pathologic aging 1. CT of brain lucencies, AJNR, 7:561-6: 1986; George, A.E., et al., Leukoencephalopathy in normal and pathologic aging: 2. MRI and brain lucencies, AJNR, 7:567-70: 1986; de Leon, M.J., George, Altered patterns of positron emission tomography glucose metabolism in Alzheimer patients with microvascular white matter disease, Am. J. Physiol., 3, 52-53): 1988; Marcus, D.L., et al., Altered glucose metabolism in microvessels from patients with Alzheimer's disease, Ann. Neurol., 26, 91-94: 1989.
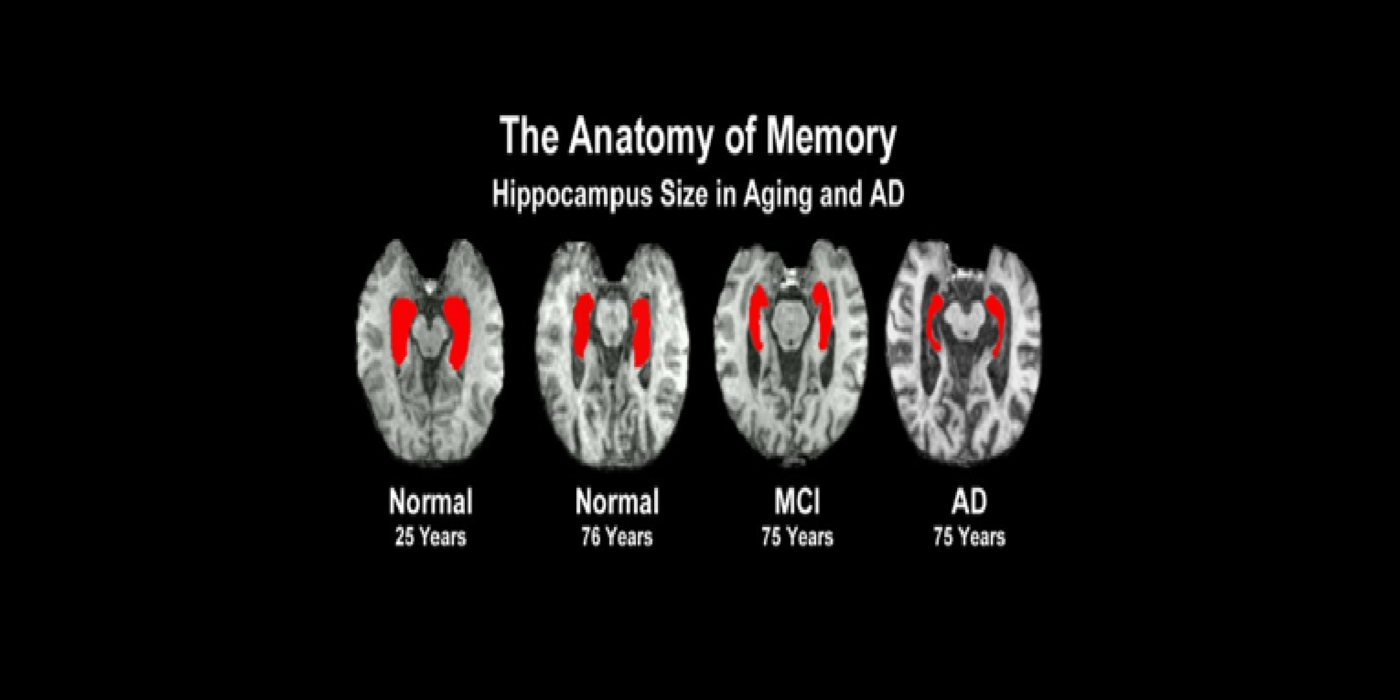
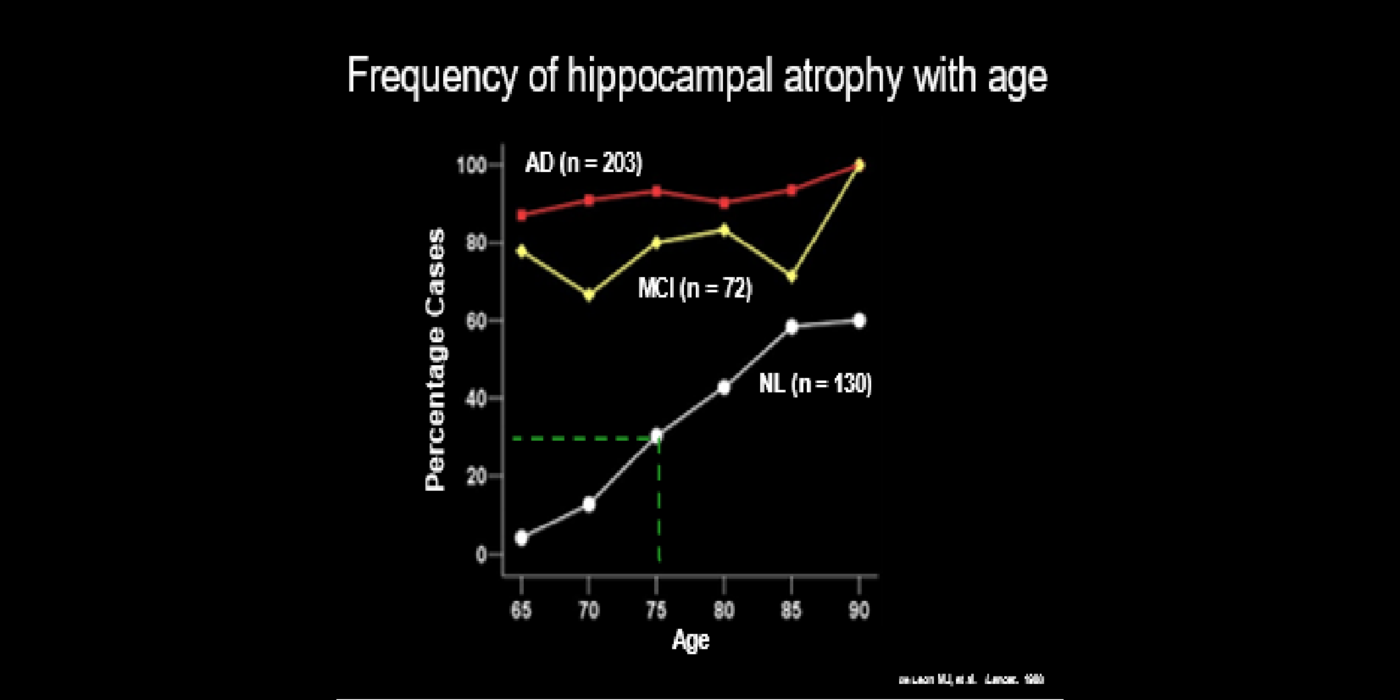
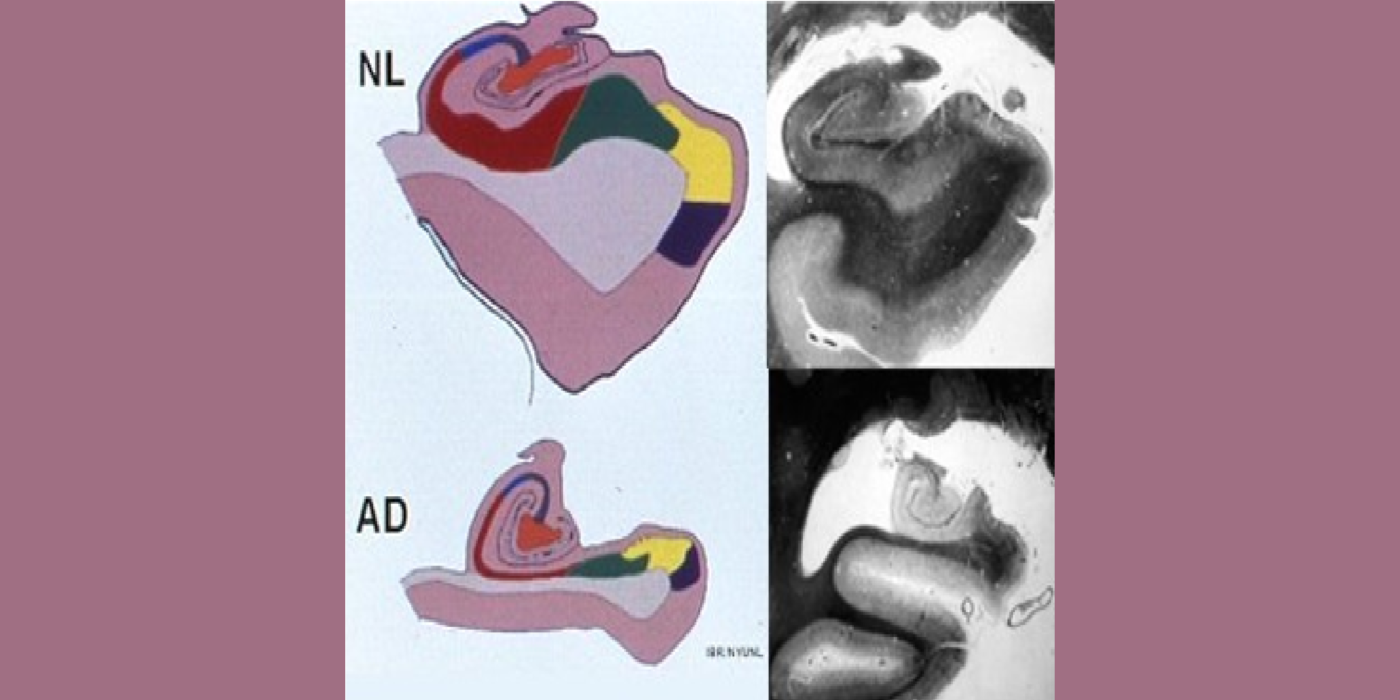
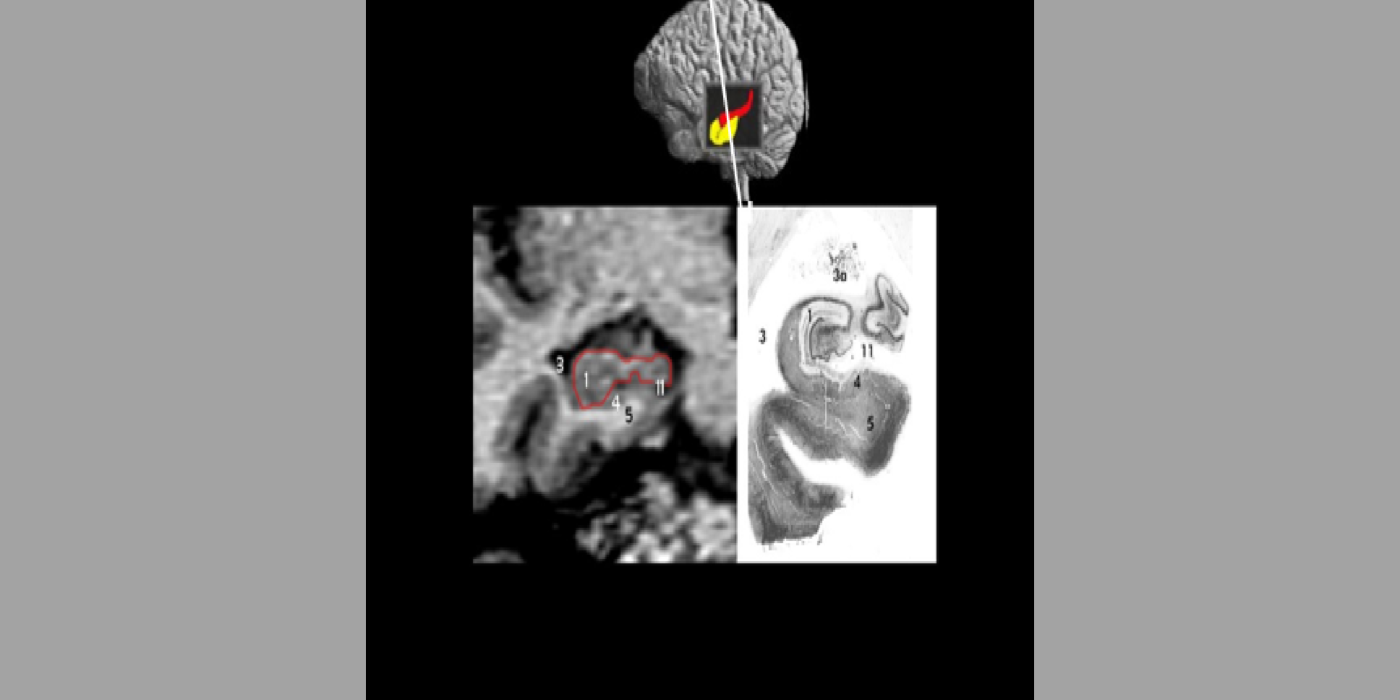
With evolving instrumentation, combined with novel image acquisition and longitudinal experimental designs, smaller targets—including atrophic changes of the hippocampus and the entorhinal cortex— allow the identification of subjects with normal cognition. But a fortuitous turning point emerged in de Leon lab ventricular anatomy studies. Using negative CT angulation to image the axis of the temporal horn of the lateral ventricle, the lab observed excess atrophic changes in the hippocampal region in subjects with AD (1988). These studies also characterized the prevalence of hippocampal atrophy in aging (30% of normal subjects with atrophy by age 75) and >80% of subjects atrophic with dementia.
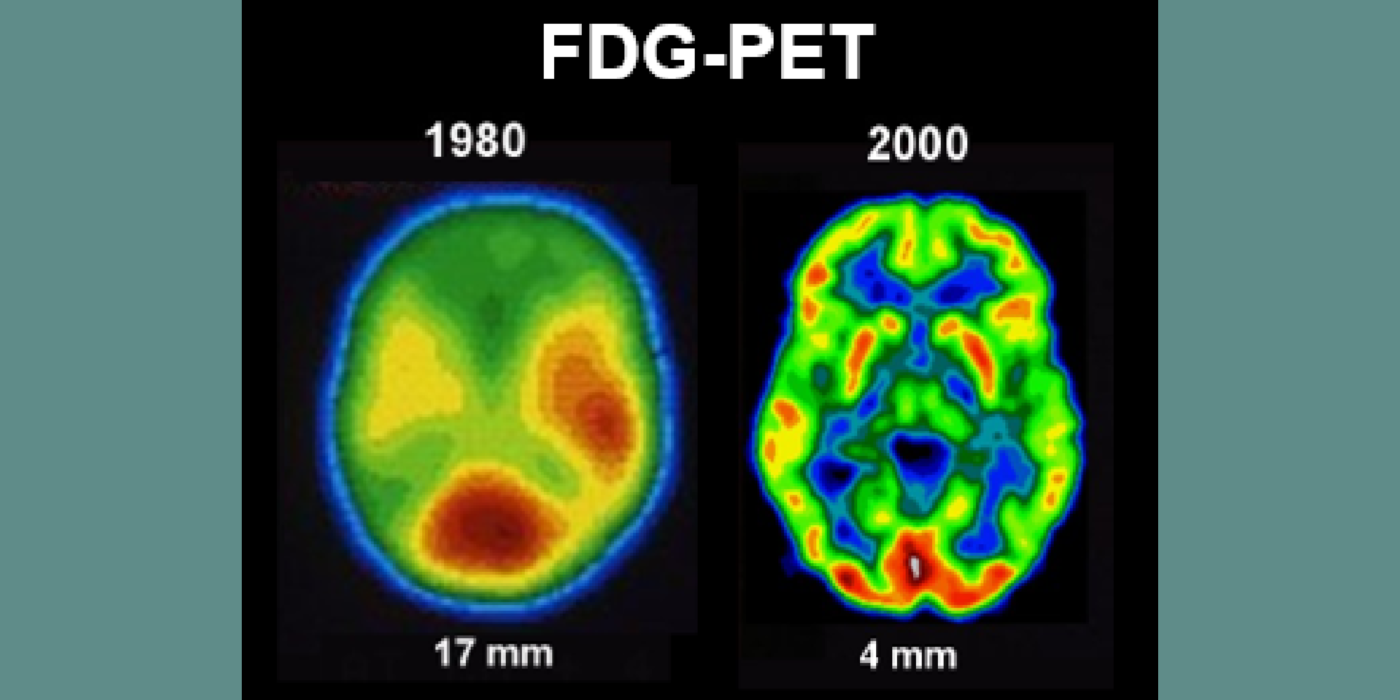
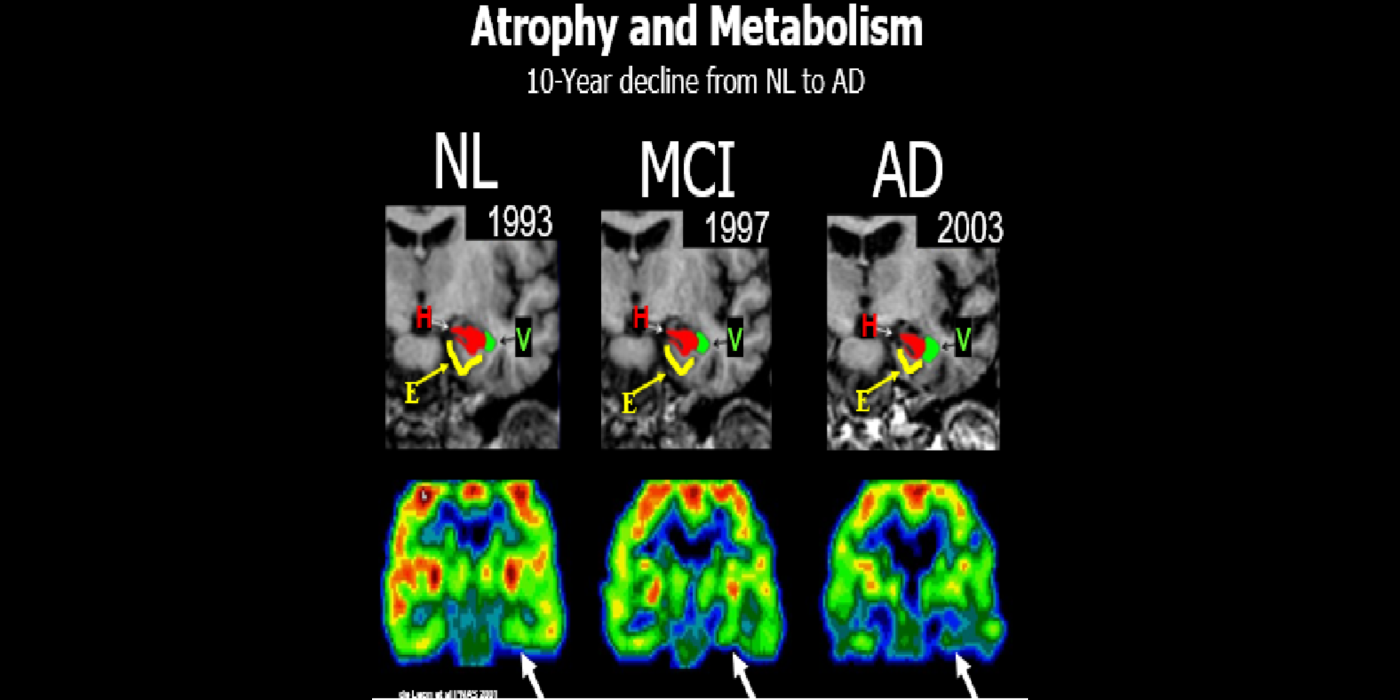
Subsequent hippocampal imaging led to the first MCI-AD prediction study (1989) and the first normal-cognition-to-MCI prediction study using MRI (high resolution (HR)) and FDG-PET (2001). This benefitted from Dr. de Leon’s collaboration with psychiatrist Barry Reisberg (the first description of MCI in 1982), and pathologist Henrik Wisniewski (the first anatomic and pathologic validations of the hippocampus in subjects with the de Leon team’s in vivo imaging). This led to the awarding, to Dr. de Leon, of The Pioneer in Imaging Award, Alois Alzheimer Centennial, Tubingen Germany, 2006. (See Carousel Images 3-6.)
Selected papers: de Leon, M.J., et al., Abnormal cortisol response in Alzheimer's disease linked to hippocampal atrophy, Lancet, 2(8607), 391-392: 1988; de Leon, M.J., et al., Early marker for Alzheimer's disease: The atrophic hippocampus, Lancet, (672-673) Sept. 16, 1989; Rusinek, H., et al., Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging, Radiology; 229(3):691-6: Dec. 2003; Bobinski M., et al., The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease, Neurosci; 95: 721-5, 2000 ; Bobinski, M., et al., MRI of entorhinal cortex in mild Alzheimer's disease, Lancet 353, 38-40: 1999.
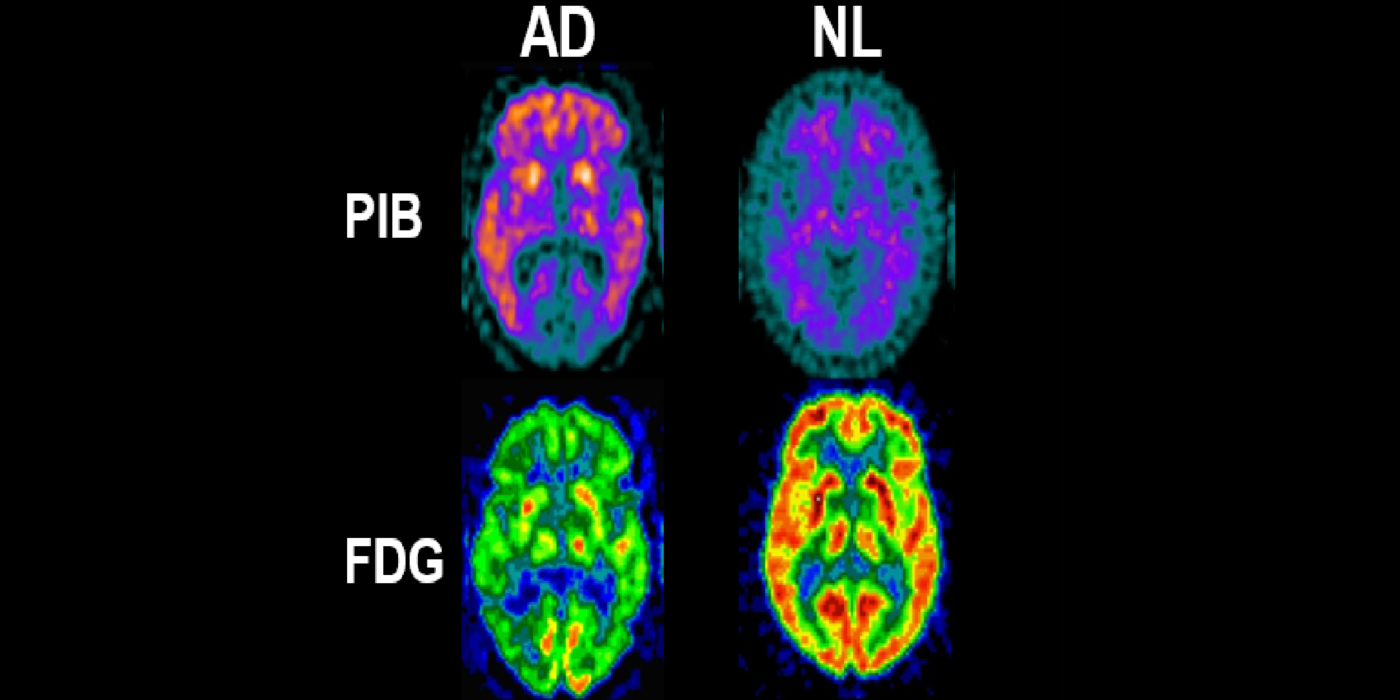
A major turning point in the AD biomarker field was first introduced by the availability of CSF biomarkers of Aβ and tauopathy and later, PET imaging biomarkers for neuropathology. These studies were revolutionary as they conferred disease specificity into the clinical exam. Plasma tests are now available. The BHII routinely recruits subjects with family AD histories, ApoE genotype, hypertension, impaired sleep, joint replacement surgery, brain trauma, and periodontal bacterial infections, and COVID-19. The BHII uses imaging, CSF, and plasma AD biomarkers in longitudinal designs to study disease progression and therapeutic interventions.
Selected papers: de Leon M.J., et al., CSF clearance in Alzheimer Disease measured with dynamic PET, J Nuc Med; 58(9):1471-1476: Sept. 2017; Glodzik, L., et al., Different Relationship Between Systolic Blood Pressure and Cerebral Perfusion in Subjects with and without hypertension, Hypertension, 73(1):197-205: Jan. 2019; Osorio,.R., et al., The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals, Neurobiol. Aging, ISSN 0197-4580, 2013; Kamer, A.R., et al., Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly, Alzheimers Demen.; 13(1):e12172: Apr. 12, 2021.
Mechanistic studies of AD
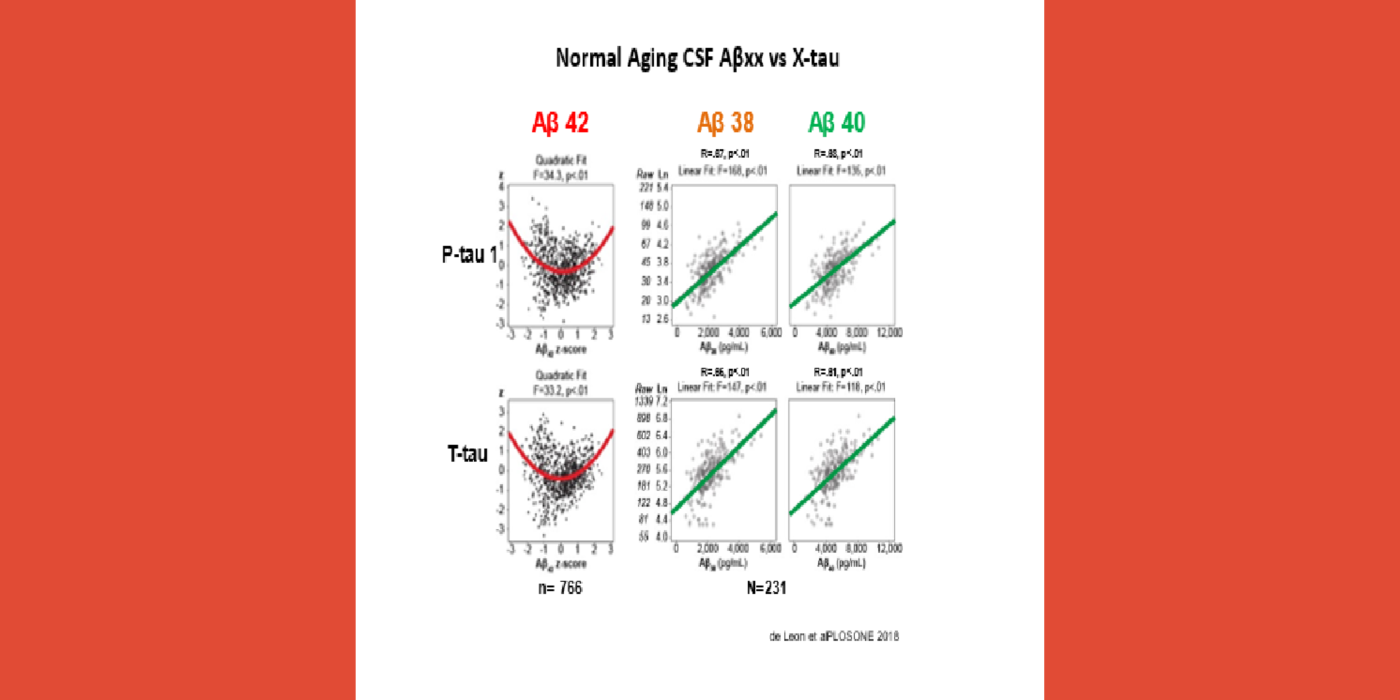
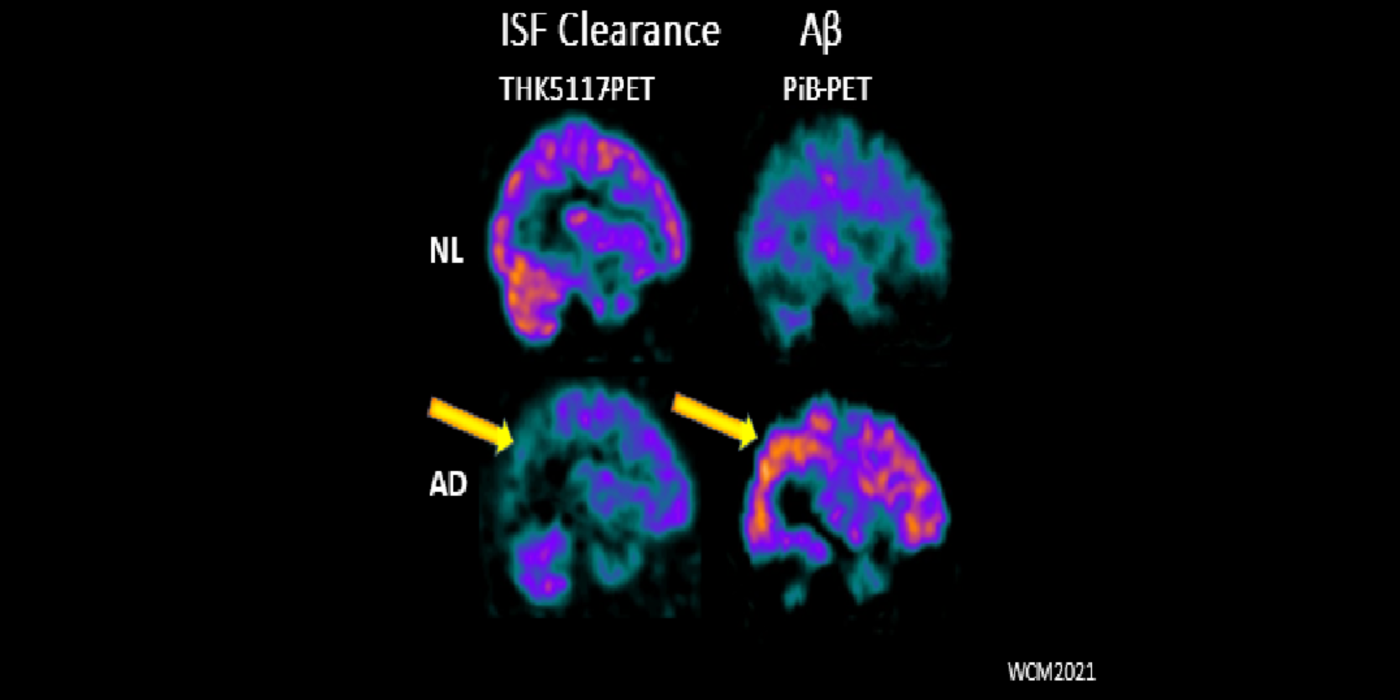
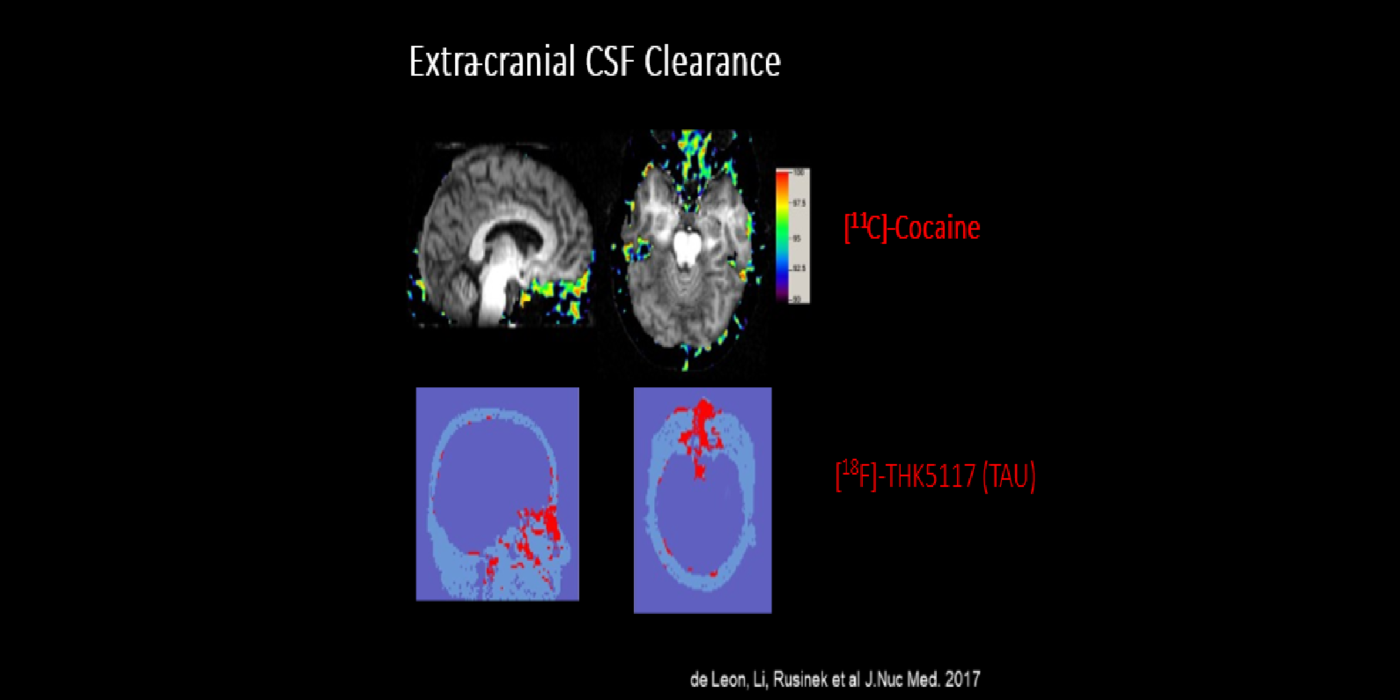
It has been hypothesized amyloid beta (Aβ) clearance is reduced in AD, potentially a causal factor for its fibrillization and accumulation. We tested the hypothesis that reduced CSF clearance was associated with amyloid accumulation using 11C-Pittsburgh compound B (PiB). We observed CSF clearance, measured from the lateral ventricle using radiotracer 18F-THK5117, was reduced in AD, and associated with the magnitude of amyloid accumulation. Little is known about the extracranial egress of CSF from the human brain. By examining the extracranial compartment with two radiotracers, we identified a nasal egress pathway through the cribriform plate into the superior nasal turbinates.
Selected papers: Tarasoff-Conway, J.M., et al., CSF clearance in Alzheimer Disease measured with dynamic PET, J. Nucl. Med.,58(9):1471-1476: Sept. 2017; Mehta, N.H., Front. Physiol., 12:769948: Jan. 4, 2022.
Collaborators
Tracy Butler, M.D.
Yi Li, M.D., Ph.D.
Anna Nordvig, M.D.
David Pisapia, M.D.
Yi Wang, Ph.D.